Element principle of chemical
Data: 2.03.2018 / Rating: 4.7 / Views: 753Gallery of Video:
Gallery of Images:
Element principle of chemical
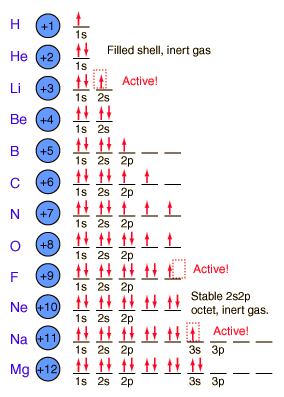
The principle objective of this paper s to introduce, through selected examples, the application of a particular finite element software code called PDEPROTRAN to mathematical models of chemical engineering systems. Periodic Table of Chemical Elements. 2019 is the International Year of the Periodic Table of Chemical Elements (IYPT 2019)! Periodic Table; Element Pins; Order your IYPT 2019 calendar from the ACS Store! Can anybody suggest how to calculate the chemical potential of an element using the first principle DFT calculation. any of the primary parts or constituents of a thing. in chemistry, a simple substance that cannot be decomposed by ordinary chemical means; elements are the basic components of which all matter is composed. Chemical elements are made up of atoms, each of which consists of a nucleus with a cloud of negatively charged electrons. The number of electrons that are gained or lost is characteristic for each element, and ultimately determines the number and types of chemical bonds atoms of that element can form. Atomic diagrams for several atoms are shown in Figure 6. Facts Date of Discovery: Known to the ancients Discoverer: Unknown Name Origin: From the Greek word arsenikos and the Latin word arsenicum Uses: Poison, conducts electricity, semiconductors Obtained From: mispickel Related Links I currently do not know of any links for Arsenic. If you do, please let me know MLA Format for Citing This Page A chemical change is one whichproduces a new substance. A state change means conversion betweenphysical states, solid liquid and gas. Chemical Laws, Concepts, and Principles. Explore the major theories, laws, and principles of chemistry, and learn how to apply them. Efflorescence Definition in Chemistry. Steam Distillation Definition and Principle in Chemistry. Carboxyl Group Definition and Examples. Chemical elements listed by electronegativity The elements of the periodic table sorted by electronegativity. click on any element's name for further chemical properties, environmental data or health effects. This list contains the 118 elements of chemistry. An element is a chemical substance made up of a particular kind of atom and hence cannot be broken down or transformed by a chemical reaction into a different element, though it can be transmuted into another element through a nuclear reaction. The weight of an atom of any given element depends on the number of protons (and neutrons) in its nucleus, but the number of protons also determines the number and arrangement of electrons that can orbit the nucleus, and it is these outer shells of electrons that. A chemical element, or an element, is defined as a material which cannot be broken down or changed into another substance using chemical means. Elements may be thought of as the basic chemical building blocks of matter. Each element is identified according to the number of protons it has in its atomic nucleus. Origin of the names of the chemical elements and multilingual dictionary of element names (72 languages); Periodic table and how the elements got their names; Process of. Chemical element A chemical element, or element, is a type of atom that is distinguished by its atomic number; that is, by the number of protons in its Firstly, there is the chemical element as the basic element, that is the abstract or transcendental element, the essence of the element, a bearer of properties Paul Dirac famously claimed this situation had been fully and completely achieved in principle: The underlying. Element Groups: Alkali Metals Alkaline Earth Metals Transition Metals Other Metals Metalloids NonMetals Halogens Noble Gases Rare Earth Elements Information about carbon14 dating. Chemical Of The Week Buckyballs; From the University of WisconsinMadison. Award winning periodic table with userfriendly element data and facts. Cool online chemistry videos, dictionary, tools, etc. a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Newlands noticed that the eighth element (fluorine, F) resembled the first (hydrogen, H), the ninth resembled the second, and so forth. His observation that every eighth element had similar properties led him to compare his chemical octaves with musical octaves, and he himself called it his law of octaves. Periodicity by octaves in chemistry suggested to him a fundamental harmony like the one. A chemical formula is a way of expressing information about the proportions of atoms that constitute a particular chemical compound, using a single line of chemical element symbols and numbers. PubChem uses the Hill system whereby the number of carbon atoms in a molecule is indicated first, the number of hydrogen atoms second, and then the. 21 Chemical Elements and Effects on Steel Mechanical Properties Steel in general is an alloy of carbon and iron, it does contain many other elements, some of which are retained from the steel making process, other elements are added to produce specific properties. Element definition is any of the four substances air, water, fire, and earth formerly believed to compose the physical universe. How to use element in a sentence. the constituents of a chemical compound; ingredient applies to any of the substances which when combined form a particular mixture. Most sulfur is, however, used in the production of sulfuric acid, which is perhaps the most important chemical manufactured by western civilisations. The most important of sulfuric acids many uses is in the manufacture of phosphoric acid, to make phosphates for fertilisers. [C13: from Latin elementum a first principle, alphabet, element, of uncertain origin element. atom (physics and chemistry) the smallest component of an element having the chemical properties of the element. allotrope a structurally different form of an element. 4 [countable a simple chemical substance that consists of atoms of only one type and cannot be split by chemical (denoting fundamental constituents of the world or celestial objects): via Old French from Latin elementum principle, rudiment All outdoor activities carry an element of risk. Chemical reactions involve the atomic. As nouns the difference between principle and element is that principle is a fundamental assumption while element is one of the simplest or essential parts or principles of which anything consists, or upon which the constitution or fundamental powers of anything are based. A chemical element is a pure substance which is composed of a single type of atom, characterized by its particular number of protons in the nuclei of its atoms, known as the atomic number and represented by the symbol Z. Chemistry Chapter 8 study guide by sloanabaker includes 98 questions covering vocabulary, terms and more. Which of the following orbital diagrams violates the Pauli exclusion principle? D Who was the first chemist to recognize patterns in chemical properties of the elements. This has a knockon effect on the outer electrons that determine an element's chemical behaviour, because the electrons feel each other's movements thanks to their electrical charges. The Chemical Abstracts Service registry number is a unique identifier of a particular chemical, designed to prevent confusion arising from different languages and naming systems. Fact box Group: 17 John Emsley unlocking the secrets of the brown element Bromine. ) Benjamin Jowett () The simplicity which is so large an element in a noble nature was laughed to scorn. Any one of the simplest chemical substances that cannot be decomposed in a chemical reaction or by any chemical means and made up of atoms all having the same number of protonsOne of the four basic building blocks of matter in theories of ancient. [C13: from Latin elementum a first principle, alphabet, element, of uncertain origin element. atom (physics and chemistry) the smallest component of an element having the chemical properties of the element. allotrope a structurally different form of an element. This simplified version of the Aufbau principle explains the structure of the periodic table of elements, where elements with similar chemical properties are listed in the same column: The chemical properties of an element depend mostly on the valence electrons located in the outermost subshell(s) which are usually the least favored. This course provides an introduction to the chemistry of biological, inorganic, and organic molecules. The emphasis is on basic principles of atomic and molecular electronic structure, thermodynamics, acidbase and redox equilibria, chemical kinetics, and catalysis. The weight of an atom of any given element depends on the number of protons (and neutrons) in its nucleus, but the number of protons also determines the number and arrangement of electrons that can orbit the nucleus, and it is these outer shells of electrons that. Two electrons fill the 1s orbital; the third electron in Li must, by the Pauli exclusion principle, occupy the next lowestenergy orbital, namely, the 2s: File: Chemical Principles Equation 9. png The fourth electron in beryllium (Be) fills the 2 s orbital, and the fifth electron in boron (B) must occupy one of the higherenergy 2 p orbitals. The law of conservation of mass (Lavoisier, 18th century): Lavoisier was one of the first to carry out quantitatively accurate chemical measurements. He demonstrated that combustion required oxygen, and he demonstrated oxygen's role in the rusting of metals. His observations led him to deduce the. PRINCIPLE OF URINALYSIS Vanngarm Gonggetyai. Routine urinalysis: Chemical examinationRoutine urinalysis: Chemical examination Protein Routine urinalysis: Microscopic examinationRoutine urinalysis: Microscopic examination Abnormal crystals Cystine Acetyl sulfadiazine. The precautionary principle shifts the burden of proof, insisting that those responsible for an activity must vouch for its harmlessness and be held responsible if damage occurs. The issues of scientific uncertainty, economics, environmental and public health protection which are embedded in the principle make this extremely complex. Principles of Reactivity: Chemical Reactions of a chemical reaction is the combination of aluminum atoms form the solid metal with bromide molecules from the liquid element to give a white solid consisting of Al 2 Br 6 molecules. Balanced chemical equation: 2Al(s) 3Br 2 (l). Atoms of one element, however, are never changed into atoms of another element as a result of a chemical reaction. The working principle of an atomic pile? An atomic pile, or more correctly a. Chemical element, also called element, any substance that cannot be decomposed into simpler substances by ordinary chemical processes. Elements are the fundamental materials of which all matter is composed. The important difference between a mixture. The atomic number (number of protons) is located right above the element symbol. Under the element symbol is the atomic mass, or atomic weight (sum of the protons and neutrons). Atomic mass is a weighted average of all naturally occurring isotopes. What determines the position of each element in the periodic table. the principle that states that the physical and chemical properties of the elements are periodic functions of their atomic numbers is. Xray Photoelectron Spectroscopy Roger Smart, Stewart McIntyre, Mike Bancroft, Igor Bello Friends Department of Physics and Materials Science chemical state of element Relative composition of the constituents in the surface region Valence band structure. a small amount of an emotion or quality: 3. a simple substance that cannot be reduced to smaller chemical parts: . any of the primary parts or constituents of a thing. in chemistry, a simple substance that cannot be decomposed by ordinary chemical means; elements are the basic components of which all matter is composed. Chemical elements are made up of atoms, each of which consists of a nucleus with a cloud of negatively charged electrons
Related Images:
- Angel eyes xvid
- Disaster movie x264
- How i met your mother 20
- 299 days war
- 2011 tinker tailor soldier spy
- Europa universalis extreme
- Wonderland secret worlds
- Max payne 2 the fall of max payne
- Beavis and butt season 2
- Yify 2007 720p
- Being human s01e05 hdtv xvid bia
- King lear 1971
- The beginners handbook of woodcarving
- Hazardous duty epub
- Dance smash 2012 vol3
- Like it big james
- HorribleSubs Akame ga Kill 1080p
- Phoenix marie house wife 1 on 1
- Alexis brill joy
- Bbc wildlife special
- Shikabane hime aka mkv
- In hell 2003
- Tom petty and the heartbreakers discography
- The best of 1930
- Crossover with crack
- Nine lives steve winwood
- 2014 nhl kings
- Andrew bird 2014
- Brooklyns Finest 2010
- Lesbian Adventures Strap On Specialists 7
- Teen wolf episode 2
- In the vip lia
- Fabri fibra tranne te
- O henrys full house
- Red hot chilli peppers stadium
- Window xp ultimate edition
- Voyage of the lonely
- Embarcadero rad studio 2010
- Windows server 2012 essential
- Barbie pop 2012
- Maya 2012 sp1
- Terra formars 05
- 24 live another day 1080 s09e01
- Fast and furious exten
- Windows 7 pt ultimate
- Originals S02E02 720p
- Windows vista
- First aid for the emergency medicine boards
- The Librarians s02
- Hubcap music seasick steve
- Air arms s410
- Ghajini 2008 sub
- Marc almond flac
- High school of the dead sub
- House of perez
- Book of songs
- Quran recitation by
- Pump it up pc
- Pro evolution soccer v60
- Schoolboy Q Remix
- Blow martin solveig
- Les annee 80
- Free download manager setup
- How to talk to anybody
- Harry potter e as relquias da morte parte 1
- C programming tutorials
- Star trek oscuridad
- Abbas kiarostami and life goes on
- Save the sound
- Jailbreak for ipod
- Express zip plus
- Eurovision 2011 final 720p
- Nfs most wanted 2005 save game editor
- Ousadia e alegria
- Tech House Top 100 September 2014
- Last samurai 2011
- 2 full movie
- Modern family s04e08 hdtv
- Windows 7 game
- S10 ghost adventures
- The beach flac
- Malcolm in the middle s2
- Hologram after effect
- Cool n smooth
- Pimp 2010 nl
- Charli xcx rules
- 1080p alexis texas









.jpg)